The representatives of the State Service of Ukraine on Medicines and Drugs Control took part in the TAIEX international seminar on the implementation of the new EU Regulation on substances of human origin (SoHO)
Опубліковано 27.11.2025 о 11:07On 19-20 November 2025 a multi-country TAIEX seminar on the implementation of the new EU Regulation on substances of human origin (SoHO) – AGR 92005 was held in Brussels (Kingdom of Belgium). Oksana Babiichuk, Deputy Head of Department on licensing of medicines manufacture, blood and certification, Head of Division on licensing of blood system entities and Kseniia Moskalenko, Chief Specialist of Division on licensing of blood system entities of Department on licensing of medicines manufacture, blood and certification took part in the event. The seminar was organized in cooperation with the European Commission’s Directorate-General for Health and Food Safety (DG SANTE) and brought together representatives from Albania, Bosnia and Herzegovina, Kosovo*, Moldova, Montenegro, North Macedonia, Serbia, Turkey and Ukraine.

Participants were presented with the requirements of Regulation (EU) 2024/1938, which sets quality and safety standards for donated blood, tissues, cells and other SoHO and replaces Directives 2002/98/EC and 2004/23/EC. The programme covered issues such as the role of competent authorities, the functioning of the SoHO Coordination Board (SCB), the authorization of SoHO establishments, EDQM and ECDC technical standards, hemovigilance and traceability, the operation of the EU SoHO platform and emergency supply planning.


During the event, experts from the European Commission and leading industry organizations provided recommendations on adapting the national legislation of participating countries to the requirements of the new Regulation and introducing modern approaches to SoHO quality and safety control.
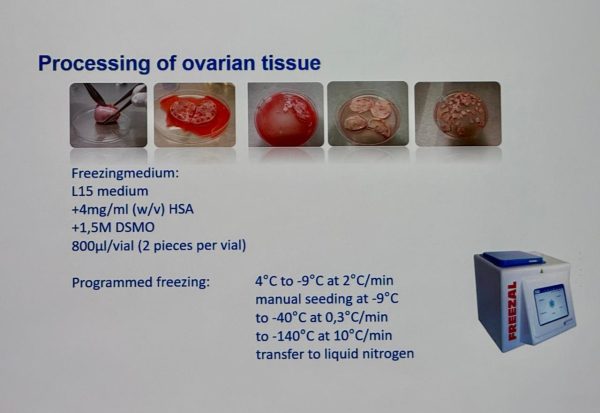
The participation of the representatives of the State Service of Ukraine on Medicines and Drugs Control in the seminar was an important step in the process of harmonizing Ukrainian legislation with European Union standards, as well as in strengthening cooperation with European partners in the field of regulating activities involving substances of human origin.
 People with visual impairment
People with visual impairment  Українською
Українською 
 Попередня
Попередня