Attention falsification!
Опубліковано 12.04.2023 о 15:00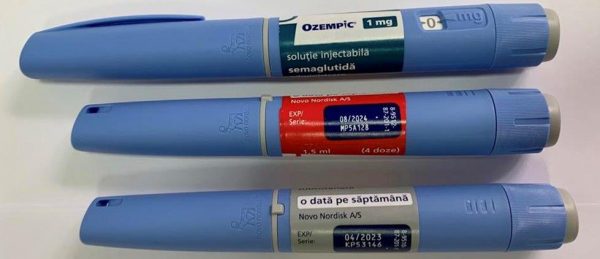
Recently, there has been an increasing of the number of reports received by the SMDC Ukraine from the regulatory bodies of EU countries and from the owners of registration certificates regarding the detection of falsified, unregistered and illegally imported medicinal products in circulation on the territory of Ukraine.
For example, on the basis of the letters of “Novo Nordix Ukraine” LLC, acting on behalf of the owner of the registration certificate A/T Novo Nordisk, Denmark (letters dated 03.24.2023 No. 109, dated 03.29.2023 No. 116) regarding detection in circulation on the territory of Ukraine of the product OZEMPIK, solution for injection, 1.34 mg/ml; 0.25 mg: 1.5 ml each in cartridges inserted into a pre-filled multi-dose disposable pen; 1 pre-filled syringe pen and 6 disposable needles of NovoFine® Plus in a cardboard box, series MP5B060, and OZEMPIK, solution for injection, 1.34 mg/ml; 1 mg: 3 ml each in cartridges inserted into a pre-filled multi-dose disposable pen; 1 pre-filled syringe pen and 4 disposable needles NovoFine® Plus in a cardboard box, series МР5В060, with the marking of production by A/T Novo Nordisk, Denmark, in English, which are falsified and have signs of falsification, the SMDC in accordance with the legislation by order of 30.03 .2023 No. 2943-001.1/002.0/17-23 prohibited the sale, storage and use of the above mentioned falsified medicinal products (orders and signs of falsification are attached).
The SMDC emphasizes that there are currently a significant number of unregistered websites offering medicines that hide the seller’s physical address, telephone number, etc., and are often the source of low-quality, smuggled and counterfeit medicines.
In order to avoid the purchase of such products, we recommend to the consumers:
- to buy medicine only on legal websites. The website of a business entity that has the right to carry out electronic retail trade of medicinal products must contain: information on the contact details of the licensing body, state quality control bodies of medicinal products; a logo with a hyperlink that is displayed on each page of the website and takes the consumer to the page of the List of business entities that have the right to carry out electronic retail trade of medicinal products (such a logo is used exclusively by pharmacies included in this List);
- do not respond to SPAM with medicines advertisements;
- beware of goods with suspiciously low prices.
If you have found a spelling error, please, notify us by selecting that text and pressing Ctrl+Enter.
 People with visual impairment
People with visual impairment  Українською
Українською 
 Попередня
Попередня