TAIEX Workshop on licensing and certification of distributors and standards for transportation and storage of medicinal products
Опубліковано 27.11.2024 о 11:42A two-day seminar has begun at the State Service of Ukraine on Medicines and Drugs Control in a videoconference format, conducted within the framework of the EU institutional building tool TAIEX in Ukraine.
TAIEX Workshop on licensing and certification of distributors and standards for transportation and storage of medicinal products was organized to facilitate EU technical assistance in the framework of the EU TAIEX institution building instrument in order to harmonize Ukrainian legislation in the sphere of state supervision (control) with the EU legislation and improve the institutional capacity of Ukrainian authorities, institutions and organizations in accordance with the European regulatory model, to support the integration of current candidate countries and potential candidates into the EU regulatory systems by harmonizing with the Acquis Communautaire in the field of regulation of medicinal products.
Employees of the central office of the State Service of Ukraine on Medicines and Drugs Control, its territorial bodies, and state enterprises are participating in the seminar.
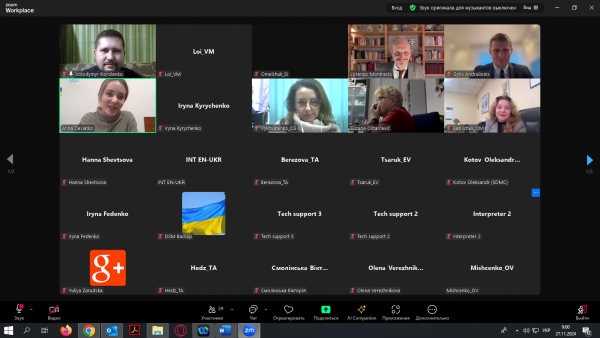
The speakers from the European side are Mr Lorenzo Montracio, representing the Italian Medicines Agency (AIFA), Mr Gytis Andrulionis, Director of the State Control Agency under the Ministry of Health of Lithuania, and Ms Suzana Ostarčević, Head of Division for safe use of medicinal products and medical devices, HALMED, Croatia.
During the event, participants will get acquainted with EU regulations on licensing and certification of distributors for compliance with GDP requirements and the experience of EU member states in modern approaches and technical support, existing rules, regulations and international practical experience in the transportation and storage of medicines.
Deputy Head of the State Service of Ukraine on Medicines and Drugs Control Volodymyr Korolenko welcomed the participants and noted that Ukraine is doing everything possible to strengthen the pharmaceutical sector and its development in accordance with the best international experience and practice, and European integration is a constant foreign policy priority.
If you have found a spelling error, please, notify us by selecting that text and pressing Ctrl+Enter.
 People with visual impairment
People with visual impairment  Українською
Українською 
 Попередня
Попередня